- D-9, Jangpura Ext., Delhi-110014
- sbsalesind@gmail.com
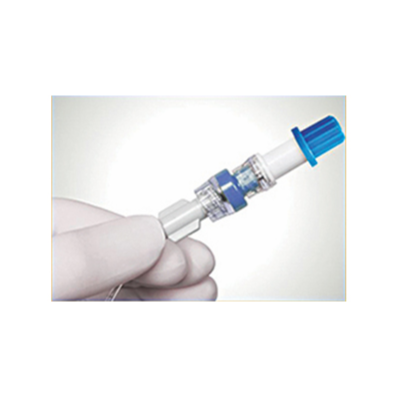
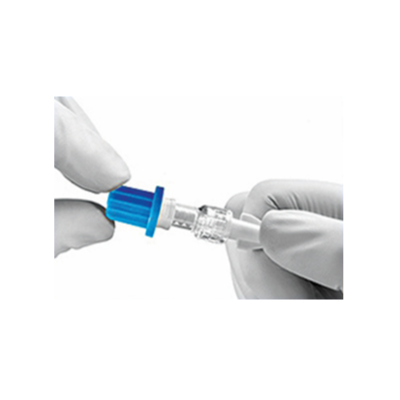






The caps are pre-filled sealed caps which are used on I.V. Access points like the hubs/ports of the catheters, needle-free connectors, drip set ends for disinfection and protection.
I.V. CAP’s are intended for use on swab-able luer access valves as a cover to protect the luer access valves from potential contamination. The product acts as a physical barrier to contamination between line accesses and serves as a disinfecting cleaner for use prior to line accesses.
I.V. CAP’s acts as a source of Continuous passive disinfection of catheter hubs & prevents contamination and bloodstream infection. The disinfectant caps have medical foam or sponge impregnated with 70% IPA or in a combination of 70%IPA &2%CHG
| Product Code | Size | Quantity | Clinical Application |
|---|---|---|---|
| 3150 | Small | 50 Pcs /Box | NA |
| 3151 | Medium | 50 Pcs /Box | NA |
| 3152 | Large | 100 Pcs /Box | NA |
| 3153 | IV Cap with 70% IPA and 2% CHG -Medium | 50 Pcs /Box | NA |
| 3154 | IV Cap with 70% IPA and 2% CHG -Large | 100 Pcs/Box | NA |